कृषि फसलों में फास्फोरस उपयोग दक्षता बढ़ाने के लिए रणनीतियां
Phosphorus (P), an essential macronutrient for all living organisms, is a vital component for building blocks of genes and chromosomes. In plants, it plays a role in virtually all biochemical processes that involve energy transfer.
It is a constituent of adenosine triphosphate (ATP), which is often termed as ‘energy currency’ of the plant cell. In plant, P is essential for photosynthesis, respiration and root growth, flowering, fruiting, seed setting and seed yield. The total P content in agricultural crops ranges from 0.1 to 0.5%.
Phosphorus is taken up by the plant as primary orthophosphate ion ( H2PO4- ) but it is also absorbed as secondary orthophosphate ( HPO42- ). Phosphorus fixation is a major problem in acid and calcareous soil. There are some techniques which helps to reduce fixation and enhances phosphorus use efficiency in soil.
Availability of Phosphorus for use by plant
Availability of P is influenced by pH as follows
- Both form of P is maximum available at the pH of 6-7.
- At pH 7.2 H2PO4-ion is equal to HPO42- .
- <7.2 pH H2PO4-ion is dominant.
- >7.2 pH HPO42- ion is dominant.
- When pH is <5 P is present as H3PO4.
- When pH is >9 PO43- is dominant.
Forms of Soil Phosphorus
The total phosphorus content in Indian soils ranges from 100 to 2000 ppm. The total phosphorus content is generally highest in the following order Granite >Genesis >Shales> Limestone > Clay. In soil both inorganic as well as organic forms of phosphorus occurred.
Inorganic Phosphorus in soil
Inorganic P constitutes four major forms of phosphorus bound to aluminium (Al-P), Iron (Fe-P), calcium (Ca-P) and Sodium (Na-P) constitutes the major active form of inorganic P. Generally Al-P and Fe-P are more abudant in acid soils; Ca-P dominates the neutral to alkaline soils and Na – P in sodic soils.
Organic Phosphorus (Po) in soil
Organic phosphorus (Po) is mainly located in fulvic acid fractions generally it accounts for 30 % to 65% of the total P in soils.Soil organic P compounds can be classified in to three groups, namely. (i) The inositol phosphates. (ii) The nucleic acids phosphates, and (iii) the phospholipids phosphates.
The Organic P can be released through mineralization processes mediated by soil organisms i.e. Phospho bacteria and plant roots in association with phosphatase secretion.
Problems in utilization of Phosphorus
Relation between soil pH and per cent distribution of soil available phosphorus
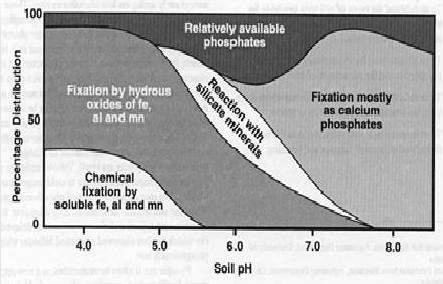
Phosphate fixation is the removal of phosphate from solution by soil which reduces the amount that plant roots can absorb. Soil pH has a deep effect on phosphate fixation.
In neutral to alkaline soils (pH 7 and above), phosphates get adsorbed on calcium carbonate and are then precipitated as tricalcium phosphate, and are slowly converted to insoluble apatites. In acid soils (pH below 7), iron and aluminium react with phosphate to form highly insoluble compounds.
Phosphorus Use Efficiency (PUE)
PUE is the amount of total biomass or yield that is produced per unit of P taken up for the crop productivity. It ranges from 10 to 20%
P uptake from source
P use Efficiency = __________________ x 100
P applied
Techniques for Enhancing P use efficiency
The technologies that have been found successful for increasing the efficiency of P use efficiency in soil.
1. Time of Phosphorus Application
Phosphorus application as a basal dressing at seeding/ planting or just prior to that, is the most widely recommended practice for seasonal crops. A split/delayed application is better for applying P under the following situations. (i) If P- fertilizer is in short supply at planning, (ii) soils are medium in available P.
2. Methods of Phosphorus Application
For higher P-use efficiency in case of conventional water – soluble P fertilizers in upland crops drilling/ placement is followed, Broadcast of P comes out as inefficient practice expect for flooded rice.
Broadcast is the simplest application method and is best suited for high- speed operations and heavy application rates. The proper band placement or drilling of P for upland crops is most important when dealing with P deficient soils and lower rates of P application.
3. Treatment of planting material with Phosphorus
Dipping the roots of rice seedlings in slurry of single super phosphate and water prior to transplanting can be followed.
It has been found that a root dip providing 10-30 kg P2O5 ha-1 can increase rice yield significantly and compares favourably with higher rates of soil applied P.
The use of 0.4% solution of MAP, DAP or filtered SSP also increases P- uptake and yields in potato.
4. Choice of fertilizer
Phosphorus fertilizer are divided in to three groups on the basis of solubility; the water- soluble group (ordinary, double, and ammoniated superphosphates, mono and diammonium phosphate), citrate soluble (dicalciumphosphate, Thomas slag, basic slag, defluorinated phosphate, and fused magnesium phosphate) and acid soluble (phosphate rock and bone meal).
The rock phosphate and bone meal applied directly in large amounts to acid soils, where the sparingly soluble phosphates are converted in to form usable by plants. When water soluble phosphorus is added to soil, part of it is taken up by the plant and the rest quickly becomes fixed in less available forms as the P reacts with other soil components.
5. Fertigation
Fertigation seems to be the best fertilizer management approach in intensive agriculture, as it is used to increase efficiency of fertilizers, increase yield, protect environment, and sustain irrigated agriculture.
Fertigation through water soluble single super phosphate (SSP) and Diammonium phosphate (DAP) enhanced the grain yield thanbroadcast.Hence, Phosphorus uptake, P use and agronomic efficiency were higher in fertigation for both the P fertilizers than broad cast method.
6. Compacted Product
While the physical mixture of phosphate rock with a soluble source of P such as Single super phosphate was considered economic and gave admirable responses, attempt was made to supply the farmers a product prepared out of compaction of phosphate rock with mono-ammonium phosphate, Single super phosphate and elemental sulphur either alone or in various combinations.
7. Phosphate Manuring of Green manure
The green manure crops upon decomposition release organic acids which not only help dissolving phosphate rocks but also complex Al, Fe in acid soils, and consequentially reduce P retention and induce greater P availability for crops.
8. Relatively high P fertilizer rate
When contrasting fertilizer calibration research performed on alkaline and calcareous soil with acid/neutral pH soil, it is apparent that relatively higher rates of broadcast/incorporated P fertilizer are required.
9. Concentrated fertilizer P bands
Adding P to soil in a concentrated band often results in further increases in yield and crop quality.
10. Application of Lime
In acidic soils Al-P is more available to upland crops than Fe-P. P can be dominantly adsorbed by Al/Fe oxides and hydroxides.
These soils are generally poor in calcium ions and, therefore, phosphates are precipitated in the form of ferric or aluminium compounds which are not so easily amenable to solubilization by plant roots or by soil microorganisms, resulting in P deficiency in plants.
Lime addition possibly brings about insoluble aluminum and iron compounds of P into more readily available and sustains phosphate – buffering action in acid soils eliminating toxicities of Al and Mn and increasing the availability of certain plant nutrients.
11. Slow release fertilizer
In alkaline soils, calcium phosphate is rapidly formed following fertilizer dissolution. A slow release P fertilizer minimizes formation of calcium phosphate as the soil solution P concentration does not spike at high levels and the P is released in a more timely fashion.
12. Balancing P with other nutrients
Adding P in combination with ammonium tends to enhance availability of both nutrients. Ammonium and other acidifying fertilizer materials can enhance P solubility and uptake by root.
13. AM (Arabuscular Mycorrhiza)
The symbiotic association between plant roots and fungi is termed as ‘mycorrhizal association’ which is known to improve the growth and yield of crops in nutrients deficient conditions. Mostly applied in P deficient soils, where the phosphorus in the vesicles diffuses out into the cytoplasm and is taken up by the plant.
Authors
Jeevika K1, Muthukrishnan R2 and K.Thamaraithuvasan3
1,2 & 3 Assistant Professor,
Vanavarayar Institute of Agriculture, Pollachi – 642103.
Corresponding author email:

